CLITHON OUALANIENSIS (Lesson, 1831), Dubious nerite snail
 |
| Photo credit: Ria Tan |
YES! The Clithon oualaniensis displays a high level of polymorphism in terms of colour and pattern of its shell [8], and no two shells are identical [26].
Table of Contents
Nomenclature
Scientific name: Clithon oualaniensis (Lesson, 1831)Synonyms:
Neritina or Theodoxus oualaniensis (Lesson, 1831)
Pictoneritina oualaniensis (Lesson, 1831)
Chinese name: 小石蜑螺 (Global Biodiversity Information Facility )
Generic name: Marine snails
Common name: Dubious nerite snail or Guamanian nerite (SeaLifeBase )
NERITE = A sea god that was transformed into a shellfish, according to Greek mythology (read the fable here)
| Animal |
||||||||||
| Mollusca |
||||||||||
| Gastropoda |
||||||||||
| Orthogastropoda |
||||||||||
| Neritaemorphi |
||||||||||
| Neritopsina (=Neritomorpha) |
||||||||||
| Neritoidea |
||||||||||
| Neritidae |
||||||||||
| Neritinae |
||||||||||
| Clithon |
||||||||||
| oualaniensis |
||||||||||
Description
Shell

Photo credit: Eunice Soh
Size
0.3 - 0.5cm [26]. It is sized and shaped like a small pea. It has a low/reduced spire [8].
Background Colour
Background colours are usually yellow, olive or greenish [28].Texture
"Smooth and glossy" [28].Patterns: The Array
The Clithon oualaniensis exhibits a wide range of patterns on its shell. The patterns can be in the form of triangles or tongues, axial (transverse), zigziag or spiral lines and patterns. The overall shell pattern is thus dependent on the visibility of the background colour and these line patterns [7]. The axial lines can differ in terms of spacing between lines, ranging from tightly arranged ones to more spaced out patterns. The tongues differ in sizes and density, where they may either be absent on the shell, or even in high densities which cover most of the shell. Some shell patterns even have axial lines and tongues that are arranged in spirals [8].| Axial pattern. Photo credit: Stella Tan |
 |
| Axial with few tongues. Photo credit: Stella Tan |
 |
| Axial with numerous tongues. Photo credit: Stella Tan |
| Black. Photo credit: Stella Tan |
 |
| Tiger pattern. Photo credit: Stella Tan |
| Giant tongue. Photo credit: Stella Tan |
| Dilution pattern. Photo credit: Stella Tan |
| Narrow spiral. Photo credit: Stella Tan |
 |
| Spiral with red-tipped tongues. Photo credit: Stella Tan |
| Spiral with black-tipped tongues. Photo credit: Stella Tan |
 |
| Ladder pattern. Photo credit: Stella Tan |
The persistence of these morphs in the C. oualaniensis populations may be attributed to apostatic selection. Predators usually formulate a search image for their prey. Thus when a particular pattern has a high frequency, it gets subjected to a correspondingly higher level of predation, resulting in a drop in its frequency. This will in turn leads to other morphs increasing in frequencies [citation needed]. However, it was noted that the shells do not look very different from each other when placed in their natural environment, albeit the shell patterns being very distinct (as seen above) [8]. Further experiments with true predators are required to invesitage this frequency-dependent selection and possible role of camouflage in this snail species.
Patterns: The Formation
The lines and tongues are formed via the overlaying of discrete white leuco-pigment and black melano-pigment stripes. During the growing process of the shell, a fine line of leuco-pigment is initially being deposited. Subsequently melano-pigment is "laid down in accurate alignment with the leuco-pigment". Tongues are formed due to the local thickening of the leuco-pigment to form a spindle-shaped, tongue-like projection [8].Patterns: The Transition
The patterns are thus determined by the physiology of these pigment-producing cells. When there is a change in the physiological state of the mantle cavity or any interruption of growth, the patterns of the shells will be affected [8].The density of tongues increases with increasing age. The older and larger the snail is, the more tongues it has towards the aperture [8].
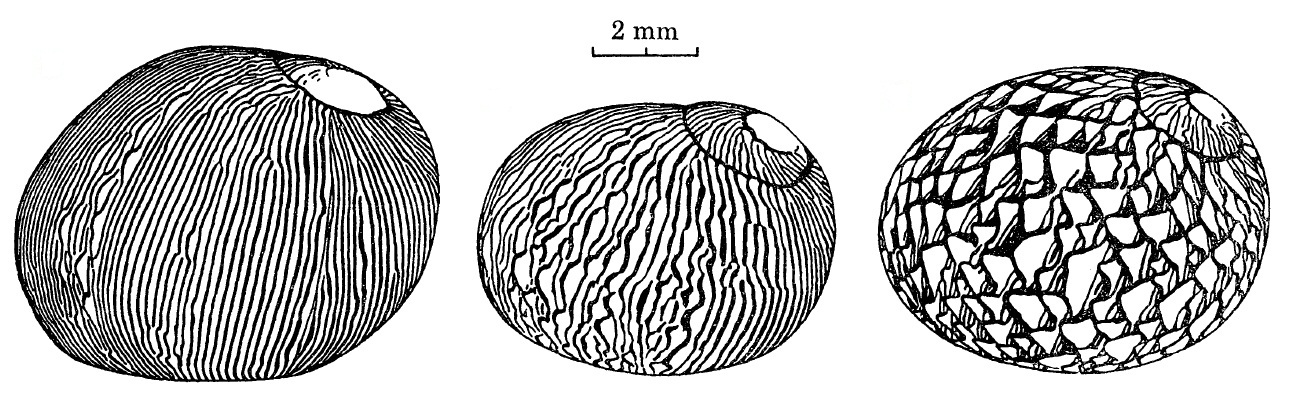 |
| Increase in tongues. Adapted from Gruneberg and Nugaliyadde (1976). |
Over time, some juveniles with axial patterns can also transit to having spiral tongues, although hypotheses on this phenomenon have yet to be tested with breeding experiments [8].
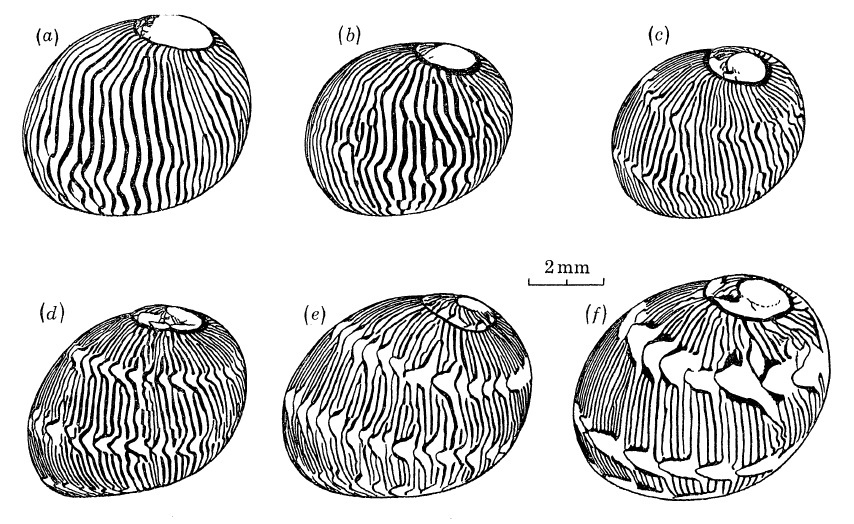 |
| Transition from axial (a) to spiral tongues (f). Adapted from Gruneberg and Nugaliyadde (1976). |
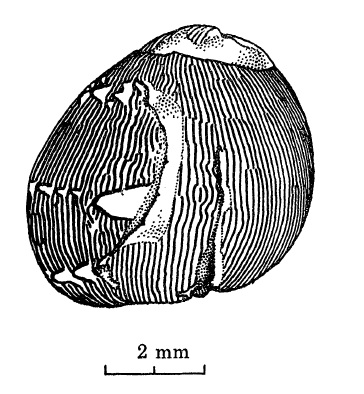 |
| Sudden change from axial to primary spiral pattern, following attempted predation. A rare event. Adapted from Gruneberg and Nugaliyadde (1976). |
Other environmental factors like predatory attacks can also lead to an abrupt change in the shell pattern. Predators like hermit crabs usually attempt to cut the snails at their aperture and pry open their operculums. When these attempts fail and they choose to give up their prey, these snails can subsequently secrete a new shell, thus causing a distinctive break in the shell pattern and a sudden change to another pattern [8].
Shell Mineralogy and Microstructure
The shell of C. oualaniensis is made up of three layers. The outer layer is calcitic, while the middle and inner ones are aragonitic. The inner layers have a crossed lamellar microstructure arrangement. They do not possess nacre, which is common in the shells of other molluscs like pearl oysters [17].Animal
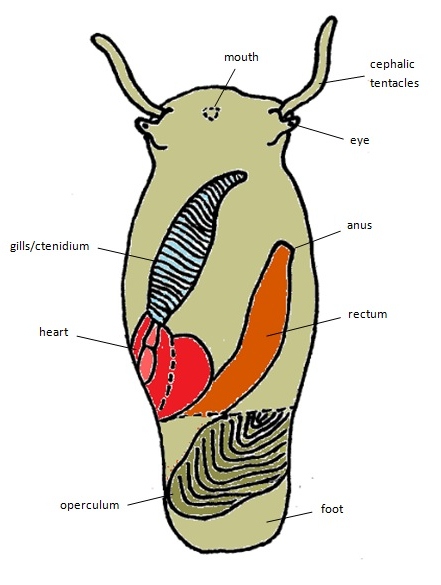 |
| General body plan (dorsal view) of a Clithon oualaniensis, removed from shell. Diagram by Stella Tan. Modified from Holthius (1995). |
Body
- Its body is "greenish grey, with black lines" [28].
- As it is a prosobranch gastropod [8], the main characteristic is that its gills are located in front of its heart.
- Its foot is large and is mainly used for locomotion [1].
- It has a pair of eyes, as well as a pair of cephalic tentacles [1].
- The operculum is semilunar or D-shaped [8]. It is usually grey with a greenish tinge [28].
- It has an open circulatory system [1].
 |
| Clithon oualaniensis with visible cephalic tentacles. Photo credit: Ria Tan |
 |
| Operculum of Clithon oualaniensis. Photo credit: Ria Tan |
Geographical Distribution
Global Distribution
The Clithon oualaniensis is widely spread in the Indo-Pacific region [8]. |
| Indo-Pacific biogeographic region. Photo credit: Eric Gaba - Wikimedia Commons user: Sting. |
These are the records of C. oualaniensis from various sources such as the National Museum of Nature and Science in Japan and the Australian Museum.
 |
| Records of Clithon oualaniensis from Global Biodiversity Information Facility (GBIF). |
According to the International Union for Conservation of Nature and Natural Resources (IUCN) [13], they are also found in these places:
| Continent |
Country |
Location |
Citations |
| Asia |
Hong Kong* |
Lung Mei Beach |
Halcrow China Ltd. (2008) [9] |
| Indonesia* |
Segara Anakan Lagoon on Java |
Nordhaus et. al (2009) [18] |
|
| Japan* |
Nagura Estuary, Ishigaki Island of the Ryuku Islands |
Ohgaki and Kosuge (2005) [20] |
|
| Peninsular Malaysia* |
Purchon and Purchon (1981) [21] |
||
| Philippines* |
Mindanao and Cebu Islands |
Borra (2006) [2] |
|
| Singapore* |
See map below |
Purchon and Purchon (1981) [21], Tan and Clements (2008) [28] |
|
| Thailand* |
Klong Wat Ta-le, Ban Mae Nam and Ban La Mai (Ko Samui district) |
Sri-aroon et. al (2005) [23] |
|
| Australia/Oceania |
Australia, Queensland* |
Dingo Beach, Nelie Bay and Champagne Bay, north of Prosperine |
Gardner et. al (1995) [6] |
| Cook Islands* |
|||
| Fiji* |
Haynes (1988) [10] |
||
| French Polynesia* |
Tahiti |
||
| Guam* |
Smith (2003) [24] |
||
| New Caledonia* |
|||
| Papua New Guinea* |
|||
| Samoa* |
|||
| Solomon Islands* |
|||
| Vanuatu* |
Unfilled rows indicate countries that require citations.
Haynes (1990) could not be accessed.
Locations from Borra (2006), Gardner et. al (1995), Halcrow China Ltd (2008), Purchon and Purchon (1981) and Smith (2003) were interpreted from the titles, as these articles could not be accessed too.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
© International Union for Conservation of Nature and Natural Resources. Source: IUCN.
Local Distribution
C. oualaniensis can be found locally at these places: Sarimbun, Punggol, Pulau Ubin, Pasir Ris, Sungei Changi, Changi North Bay, Tanah Merah, South Changi, Sungei Bedok, Marina East, Kallang Basin and Tuas South [28]. |
| Local distribution of Clithon oualaniensis. Picture of giant tongue pattern used to represent local Clithon oualaniensis populations. Diagram by Stella Tan. |
Biology
Habitat
Clithon oualaniensis snails are often found in either brackish or freshwater habitats. They are known to have brackish water origin and |
| Clithon oualaniensis on seagrasses. Photo credit: Ria Tan |
They are usually found in huge numbers "near monsoon drains, among seagrasses and seaweeds" (wildsingapore), "mangrove streams, muddy sand banks" [28]. They can also be found in and estuaries (SeaLifeBase). In general, they seem to prefer "slow-moving, sheltered and shallow waters." [26].
They are also able to rapidly colonize artificial habitats, such as that of sea walls [28].
Behaviour
C. oualaniensis snails are usually seen at highest density right after the tide receded. This phenomenon happens "regardless of day and night" [19]. |
| Clithon oualaniensis seen during daytime. Photo credit: Ria Tan. |
 |
| Clithon oualaniensis seen during nighttime. Photo credit: Ria Tan. |
Ohgaki (2001) studied the activity pattern of C. oualaniensis, whereby he counted the number of visible snails during a 24-hour period. Many were seen to partially bury themselves in the sand during the advent of rising and receding tides.
 |
| Partially buried shells. Photo credit: Kok Sheng. |
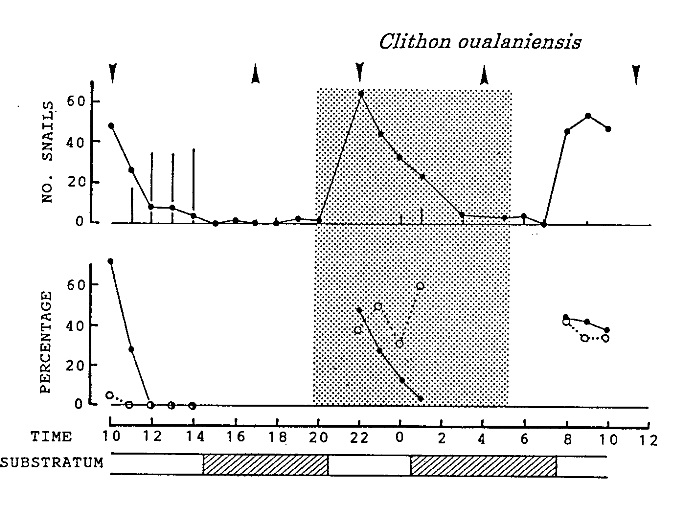 |
| Activity pattern of Clithon oualaniensis during the 24h observation. An upward wedge represents time of high tide and a downward wedge represents time of low tide. A half-toned area indicates dark period between sunset and sunrise. Adapted from Ohgaki (2001). |
When the tide just recedes, they are likely to be coming out of the sand to start a feeding frenzy as their preferred food of detritus and algae [25] will be washed up shore.
When the tide rises, they burrow into the sand/substrate. This reduces their visibility to underwater predators and lowers the chances of being washed away by the waves [19].
 |
| Or even multiple! Photo credit: Ria Tan. |
Pairing of snails (i.e attachment of the snail to another shell) is a common sight during low tide. Such pairings only occur between conspecifics, even though there might be other closely related
 |
| Pairing of Clithon oualaniensis. Photo credit: Wj. |
 |
| Occassionally you do see double pairings. Photo credit: Ria Tan. |
Reproduction
As opposed to more primitive gastropod groups (Patellogastropoda and Vestigastropoda), gastropods in the Neritimorpha always show internal fertilisation [4]. The male inserts the penis into the vagina opening for deposition of spermatophore.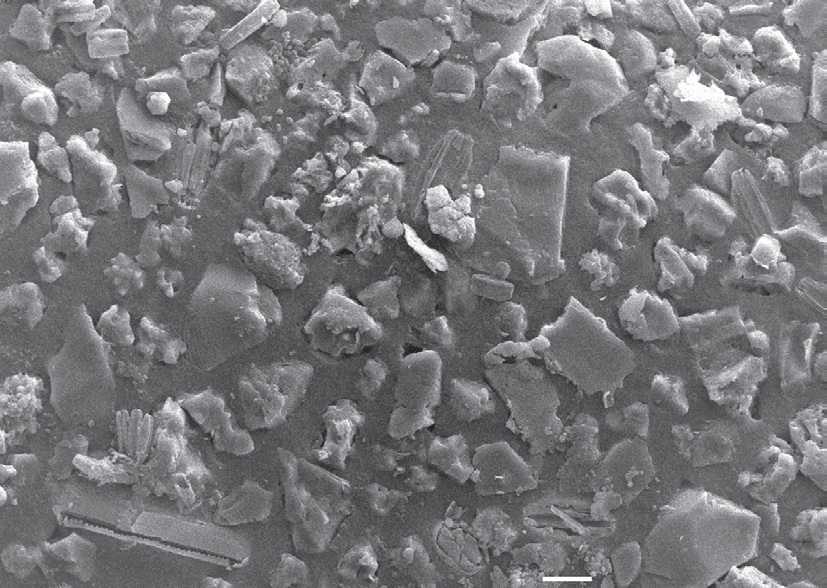 |
| Surface feature of Clithon oualaniensis egg capsule. Scale: 10µm. Adapted from Tan and Lee (2009). |
*More information is required.
C. oualaniensis is oviparous. For all internally fertilizing gastropods, they have a leathery egg capsule to protect the developing embryos [1]. These egg capsules are very small, only having a greatest diameter of 1.1mm. They are "dome-shaped with an oval outline". The diagram on the right shows the external surface of the capsule. It comprises of differing sizes of sand grains and diatom frustules. This is strikingly different from the egg capsules of other Nerita species, which posses spherulites instead. This difference reveal a diversified adaptation to the intertidal habitats. This feature can also be used as a distinguishing character to identify between neritid species [27].
Life Cycle
The completion of the life cycle of C. oualaniensis is partially dependent on other animals of the same species. Their egg capsules need to be attached to the shell of that other animal. Within the egg capsule, the veliger larvae will develop into a juvenile. Subsequently juvenile snails with complete shells will emerge [8].*More information is required.
Threats to Conservation
Status:"Least Concerned" in the IUCN Red List of Threatened Species [14]
"Vulnerable" in the Red List of Threatened Animals of Singapore [5]
Habitat Loss and Degradation
This might be due to localised habitat modifications such as reclamation (NParks Flora and Fauna Web, [16]) that resulted in massive habitat loss for Clithon oualaniensis populations [28].An example of a local habitat modification would be the Marina South shore which was transformed into a freshwater body in 2010 (Public Utilities Board, 2012). The change in water salinity might have led to the demise of local populations of C. oualaniensis. Although this could only be a speculation, studies on the effect of salinity on C. oualaniensis had indeed discovered that a drop in salinity resulted in a significant reduction of the snail's activities [25].
Overcollection
The snails have also been exploited for sale:1. These online websites are only some of the many existing examples that sell C. oualaniensis shells.
 |
| Clithon oualaniensis on ebay. |
 |
| Clithon oualaniensis on Schooner Specimen Shells. |
![Cooked_Snail_Found_In_Rajang_River[1].jpg Cooked_Snail_Found_In_Rajang_River[1].jpg](files/Cooked_Snail_Found_In_Rajang_River%5B1%5D.jpg) |
| Cooked nerites from Rajang River, Sarawak, Malaysia. Photo credit: Kinglaw, Wikimedia Commons User, 2008. |
2.They are also used to make accessories like necklaces (NParks Flora and Fauna Web, [16]).
3. There are some nerites found to be cooked as food in Sarawak, Malaysia. Clithon species are edible and hence are usually sold in the markets. This is a speculated source of threat to the nerite snail populations. The public also needs to be warned of the accumulation of heavy metals in these snails that might deem them unfit for consumption [15].
There is hence a call for marine protected areas, and more stringent legislature to deter over-collection threats (NParks Flora and Fauna Web, [16]).
Species Diagnosis
There are many species of neritids that are polymorphic [7]. Hence the use of shell shape and patterns for identification might be ambiguous and inaccurate. The analysis of secondary sexual characteristics is thus a more recommended strategy for species diagnosis, as neritid species exhibit notable variations [11].
Between Male and Female
The sexes are separate in Clithon [8]. In other words, they are dioecious.The male penis is located at the right side of its anterior head region. They also have a pouch where the penis can be "tucked away" [11] when not in use.
Females, on the other hand, do not posses a penis or a pouch [11].
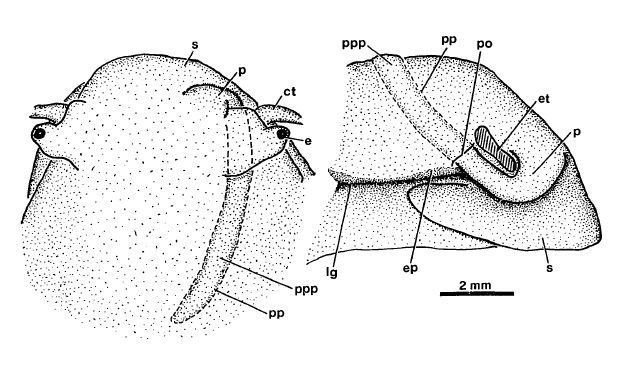 |
| Penis stored in pouch of a male Clithon oualaniensis. Dorsal (left) and right (right) views. Identical pouches in C. sowerbyana and C. chlorostomus. Ct, cephalic tentacle; e, eye; ep, epipodium; et,eye stalk and tentacle base, cut away; lg, lateral groove; p, penis; po, opening of penis pouch; pp, outline of penis pouch; ppp, penis in penis pouch; s, snout. Diagram adapted from Holthius (1995). |
| Right side view of female, penis and penis pouch absent. Adapted from Holthius (1995). |
Between Related Taxa
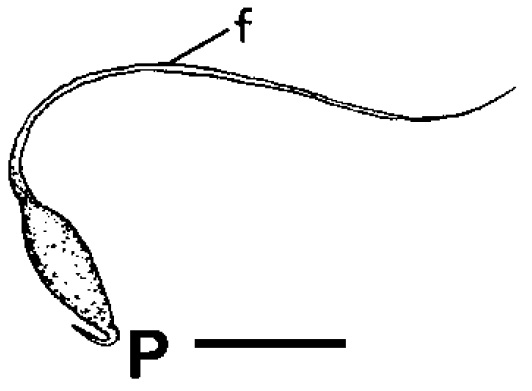 |
| Spermatophore of Clithon oualaniensis. Adapted from Haynes (2005). |
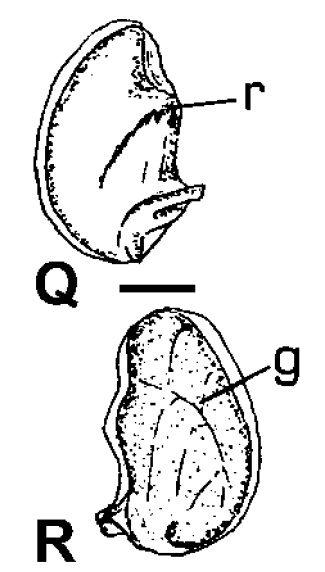 |
| Inner (Q) and outer (R) view of the operculum of Clithon oualaniensis. Scale: 1mm. g groove, r ridge. Adapted from Haynes (2005). |
In all Clithon species, a groove is present on the outer surface of the opercula. This groove in turn corresponds with a ridge on the inner surface. This groove (ridge) characteristic is not found in Neritina species [11].
*Look at Holthius (1995) [12] for more detailed morphological comparisons between related genus.
*Information on distinguishing between species to be updated in the future.
As an aid for biologists to identify various organisms, taxonomic keys come in handy. Below is an example of a taxonomic key that was used to identify C. oualaniensis among gastropods present in the Pulicat Lake, India [22]:
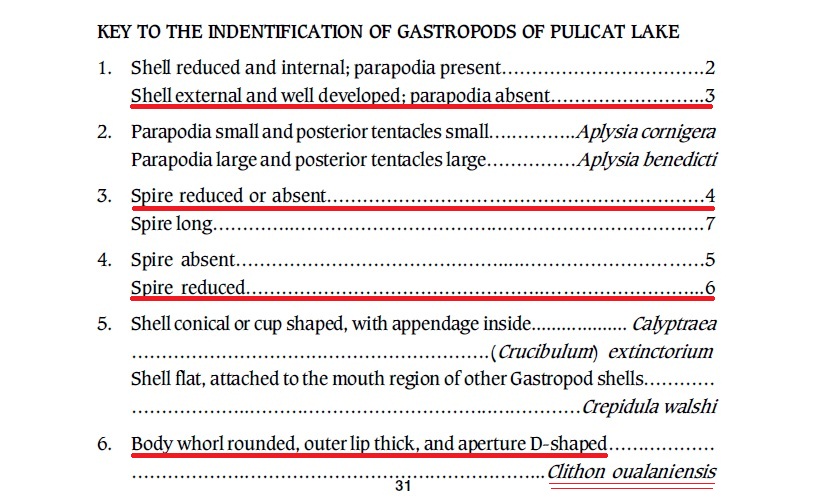 |
| Taxonomic key of Clithon oualaniensis. Adapted from Sanjeeva Raj (2006). |
Type Information
The holotype of Clithon oualaniensis was from Ovalau, Fiji and now placed in the Museum National d'Histoire Naturelle, Paris. The holotype is the original specimen used to describe the species. It is important as it possesses diagnostic characters and features, and thus serve as a point of reference for future species identification. There are voucher specimens of C. oualaniensis kept at the Australian Museum, Sydney too (voucher number: C436889) [11].
Classification Information
Below is a phylogenetic tree of Gastropoda, which consists of 2 main clades: ((Vetigastropoda + Patellogastropoda) + (Cocculinida + Neomphalina)) and (Neritimorpha + Apogastropoda) [1]. Neritidae is the taxon group that Clithon oualaniensis falls under.
 |
| Phylogeny of Gastropoda. Diagram adapted from Aktipis (2009). |
Neritimorpha (=Neritopsina) has 3 clades: Cycloneritimorpha, Cyrtoneritimorpha and Palaeozoic Neritimorpha. Only Cycloneritimorpha is extant, the other two have all members of the group being extinct. Cycloneritimorpha consists of four groups: Helicinoidea, Hydrocenoidea, Neritoidea and Neritopsoidea. Neritidae is nested within Neritoidea.
Neritidae has five subgroups: Neritinae, Neritininae, Smaragdiinae, Neritariinae and Velatinae (the last two being extinct) [3].
It is interesting to note that the group Neritidae consists of species living in various habitats: those on intertidal and supratidal rocks (eg Nerita), in brackish- and fresh- water (eg Theodoxus, Clithon, Neritina and Septaria) as well as in shallow subtidal waters (eg Smaragdia) [13].
Taxonavigation
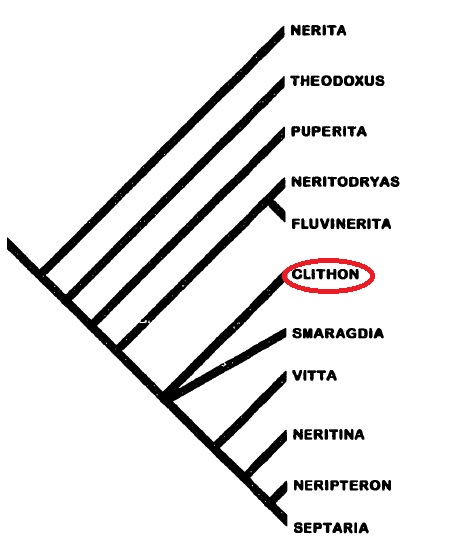 |
| Phylogeny of Neritidae. Adapted from Holthius (1995). |
Related species:
Clithon bicolor (Récluz, 1843)
Clithon chlorostoma
Clithon corona (Linné, 1758)
Clithon coronata (Leach, 1815)
Clithon diadema (Broderip, 1832)
Clithon faba (Sowerby, 1836)
Clithon francoisi (Nabille, 1895)
Clithon glabrata (Sowerby, 1849)
Clithon olivaceus (Récluz, 1843)
Clithon pritchardi (Dohrn, 1861)
Clithon spinosus (Sowerby, 1825)
Clithon squrrosus (Récluz, 1843)
Clithon longispina (Récluz, 1841)
(encyclopedia of life) and [11]
Holthius (1995) analyzed 57 character states (i.e morphological characters such as male and female reproductive systems, operculum etc) in a character matrix.
Result: A single most parsimonious tree was constructed to show the phylogenetic position of the genus Clithon. Such phylogenetic trees reflect the evolutionary history of the other taxa groups related to Clithon, and show how they have speciated to form a monophyletic group with distinctive morphological characterisitcs. Holthius have also done further analyses on adaptations to respective their habitats (such as adaptations in kidney functions of Clithon in both brackish and freshwater environments, the key driving factor that allowed them to invade and survive in freshwater) [12].
Further molecular phylogenetic analysis is recommended for the Clithon subgroup to better understand the phylogenetic position of Clithon oualaniensis.
Related Links
The Digital Nature Archive of Singapore. 2012. Clithon oualaniensis. Raffles Museum of Biodiversity Research.
Wildsingapore. 2008. Dubious nerite snails. Wild Fact Sheets.
Further Readings
Goodhart, C.B. 1987. Why are some snails visibly polymorphic, and others not? Biological Journal of the Linnean Society, 31: 35-38.
Gruneberg, H. 1979. A search for causes of polymorphism in Clithon oualaniensis (Lesson) (Gastropoda; Prosobranchia). Proceedings of the Royal Society of London. Series B, Biological Sciences, 203(1153): 379-386.
Gruneberg, H. 1980. On pseudo-polymorphism. Proceedings of the Royal Society of Longon, Series B, Biological Sciences, 210(1181): 533-548.
Literature cited/References
[1] Aktipis, S. W. 2009. Clarifying gastropod phylogenies: Exploring deep relationships among vetigastropoda and related taxa. Harvard University, UMI Dissertations Publishing.[2] Borra, R.G. 2006. Geometric morphometric and qualitative analysis of the shell in some collected species of marine, freshwater and land snails. Msu-llingan Institute of Technology, 9200 lligan City, Philippines.
[3] Bouchet, P. and Rocroi, J.P. 2005. Classification and nomenclator of gastropod families. International Journal of Malacology, 47(1-2), 397 pp.
[4] Calvo, M and Templado, J. 2005. Spermatophores of three Mediterranean species of vermetid gastropods (Caenogastropoda). Journal of Molluscan Studies, 71(3): 301-303.
[5] Davison, G.W.H., Ng, P.K.L. and Ho, H.C. 2008. The Singapore Red Data Book: Threatened plants and animals of Singapore. The Nature Society of Singapore.
[6] Gardner, M.M, Williamson, P.B.I. and Hughes, J.M. 1995. The relationship between shell-pattern frequency and microhabitat variation in the intertidal prosobranch Clithon oualaniensis (Lesson). Malacologia 36:97-109.
[7] Gruneberg, H. 1982. Pseudo-polymorphism in Clithon oualaniensis. Proceedings of the Royal Society of London, Series B, Biological Sciences, 216(1203): 147-157.
[8] Gruneberg, H. and Nugaliyadde, L. 1976. Population studies on a polymorphic prosobranch snail (Clithon (pictoneriti) oualaniensis lesson). Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 275(940): 385-437.
[9] Halcrow China Ltd. 2008. Supplementary Information on Additional Extensive Intertidal Surveys.
[10] Haynes, A. 1988. The gastropods in the streams of five Fiji islands (Vanua Levu, Ovalau, Gau, Kadavu, and Teveuni). The Veliger, 29: 204-210.
[11] Haynes, A. 2005. An evaluation of members of the genera Clithon Montfort, 1810 and Neritina Lamarck 1816 (Gastropoda: Neritidae). Molluscan Research, 25(2): 75-84.
[12] Holthuis, B. V. 1995. Evolution between marine and freshwater habitats: A case study of the gastropod suborder Neritopsina. University of Washington. ProQuest Dissertations and Theses, 1-287.
[13] Kano, Y., Chiba, S. and Kase, T. 2002. Major adaptive radiation in neritopsine gastropods estimated from 28S rRNA sequences and fossil records. Proceedings of the Royal Society, Biological Sciences, 269(1508): 2457-2465.
[14] Köhler, F. 2011. Clithon oualaniensis. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. <www.iucnredlist.org>. Downloaded on 04 November 2012.
[15] Lau, S., Mohamed, M., Tan, C.Y.A. and Su'ut, S. 1998. Accumulation of heavy metals in freshwater molluscs. Science of the Total Environment, 214: 113-121.
[16] National Parks Board. 2010. NParks Flora and Fauna Web. Retrieved from: http://florafaunaweb.nparks.gov.sg/special-pages/Record-List.aspx?OptionId=1203&DataSourceIDNo=2&CatN=TaxonomicGroup&CatO=Mollusc on 08 November 2012.
[17] München, A.N., Praha, J.F., Yancey, T.E. , Station, C. and Dunwoody, J.R.A. 2007. Larval shells of late Palaeozoic naticopsid gastropods (Neritopsoidea: Neritimorpha) with a discussion of the early neritimorpha evolution. Pälaontologische Zeitschrift, 81(3): 213-228.
[18] Nordhaus, I., Hadipudjana, F.A., Janssen, R. and Pamungkas, J. 2009. Spatio-temporal variation of macrobenthic communities in the mangrove-fringed Segara Anakan lagoon, Indonesia, affected by anthropogenic activities. Regional Environmental Change, 9: 291-313.
[19] Oghaki, S. 2001. An example of activity pattern of the estuarine snails, Clithon faba and Clithon oualaniensis (Gastropod: Neritidae) in the Nagura Lagoon, Ishigaki Island. Argonauta, Kansai Marine Biological Seminar Series, 4: 28-37.
[20] Ohgaki, S. and Kosuge, T. 2005. A circa-decadal change in the gastropod fauna on a tidal flat in an island mangrove estuary. Zoological Science, 22: 49-56.
[21] Purchon, R.D. and Purchon, D.E.A. 1981. The marine shelled mollusca of West Malaysia and Singapore. Part 1 General introduction and an account of the collecting stations. Journal of Molluscan Studies, 47: 290-312.
[22] Sanjeeva Raj, P.J. 2006. Macro fauna of pulicat lake. NBA Bulletin No. 6, National Biodiversity Authority, Chennai, Tamil Nadu, India.
[23] Sri-aroon, P., Lohachit, C. and Harada, M. 2005. Brackish-water mollusks of Surat Thani Province, Southern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health, 36(4): 180-188.
[24] Smith, B.D. 2003. Prosobranch gastropods of Guam. Micronesica, 35-36: 244-270.
[25] Takada, Y. 2000. Activity patterns of Clithon oualaniensis (Mollusca: Gastropoda) on intertidal seagrass beds in Hong Kong. Hong Kong University Press. 225 pp.
[26] Tan, L.W.H. and Ng, P.K.L. 1988. A Guide to Seashore Life. The Singapore Science Centre, Singapore.
[27] Tan, K.S. and Lee, S.S.C. 2009. Neritid egg capsules: are they all that different? Steenstrupia 30(2): 115-125. Copenhagen, Denmark.
[28] Tan, S.K. and Clements, R. 2008. Taxonomy and distribution of Neritidae (Mollusca: Gastropoda) in Singapore. Zoological Studies, 47(4): 481-494.